Uncovering the Impact of Exercise on Pulmonary Vascular Metabolism
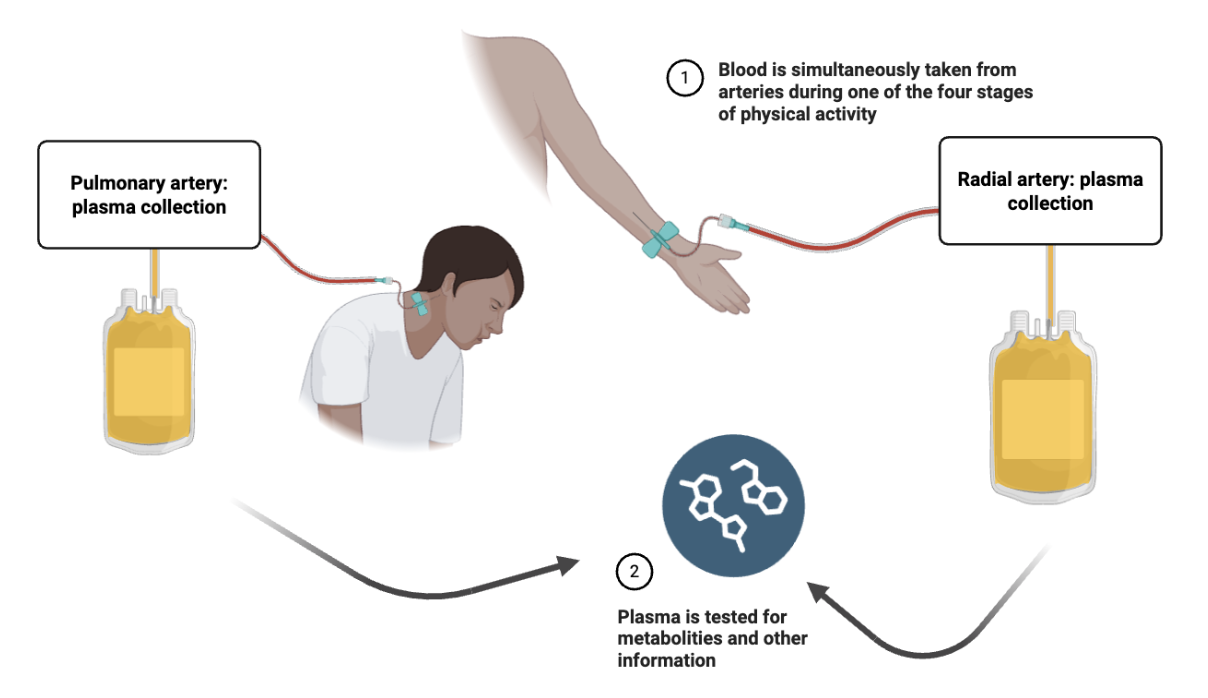
Diagram showing simultaneous blood collection from the pulmonary artery and radial artery during one of four stages of physical activity. Plasma from both sites is collected into separate containers. The plasma is then tested for metabolites and other information.
By Lexi Bean
What is the study about?
Your pulmonary arteries carry deoxygenated blood from your heart to your lungs. In pulmonary arterial hypertension (PAH), the pressure in these arteries is too high. This makes it harder for blood to flow through the lungs; over time, that can lead the heart to weaken. Exercise should cause the pulmonary arteries to expand, accommodating more blood flow. In PAH patients, this doesn’t happen; however, the specific metabolic pathways involved in this dysregulated process remain unknown.
One key indicator of PAH is excessive growth of cells that thicken blood vessel walls. Metabolic signals are believed to induce this growth. Therefore, it is crucial to research the role of metabolic pathways, or chemical reactions that break down molecules for energy. So, the question becomes: how do we identify specific metabolic pathways and their involvement in this disease?
This process is present in all types of PAH, including connective tissue disease associated PAH (CTD-PAH). Connective tissue diseases are disorders that affect the tissues that support and connect the body’s organs and other structures. CTD is caused by genetic mutations that affect protein assembly, which leads to loss of structural integrity and the weakening of blood cells. Patients with CTD-PAH tend to have the worst symptoms, which is why the researchers decided to start here.
Dr. Brian Graham and Dr. Michael H. Lee’s lab at UCSF published a 2022 study which found dysregulated pulmonary vascular metabolism in CTD-PAH. Now, in their 2025 study, they expand on this knowledge, aiming for breakthroughs on the clinical applications of how this dysregulated metabolism affects CTD-PAH.
How was it conducted?
So, how is this study different from prior work on metabolic pathways? First, PAH patients’ symptoms usually worsen during physical exercise; however, due to patient risk, most patients participate at rest. Therefore, little is known about how the metabolites (small molecules involved in metabolism) in the blood changes with exercise. Many previous studies also use single-site sampling, or collecting all blood samples from just one spot in the body. However, blood moves through the lungs, and therefore, this approach can lead to overlooking important changes in metabolism in the bloodstream.
The methodology used in this study took a different approach, with a team of experienced physicians comparing blood samples from both the pulmonary artery (before blood passes through the lungs) and the radial artery in the arm (after blood has passed through the lungs). This approach is necessary because the blood pressure is so high in these patients that it is unsafe to take a biopsy. This dual sampling approach allows us to ensure that the changes we are seeing are due to changes in the lungy’s biology and not the biology of other muscles or organs.
For this study, researchers recruited participants from São Paulo, Brazil, with both a) a CTD and b) diagnosed or suspected PAH. 63 patients had their heart and lung function measured while doing different levels of exercise: resting, light pedaling, peak exercise, and recovery. At each level, blood was taken from both arteries and analyzed to measure hundreds of metabolites. The researchers’ main goal was to identify specific metabolic markers of CTD-PAH and relate them to physiologic, clinical parameters. In other words, the researchers predicted that the lung vessels’ biology would change throughout the study based on the degree of physical excursion.
What did they discover?
The researchers had many key findings about the effects of exercise on metabolic patterns. They found interesting roles of different molecules and pathways that can indicate disease. For the readers who want a deeper glimpse of the findings, here are some specifics:
Lactate: Lactate is a molecule formed as a product of glucose breakdown and is mainly present when oxygen supply is limited. The researchers found that at peak exercise, the lungs may rely more on lactate for energy. Based on this finding, the researchers propose that it is crucial to assess the effectiveness of glycolysis-suppressing interventions during high exercise levels, as well as the role of lactate in CTD-PAH disease development.
Tryptophan-kynurenine metabolic pathway: Using heat maps, the researchers looked at this pathway over the full course of exercise (resting to peak activity to recovery). They found increased volume of both tryptophan metabolites and carnitines, nutrients that turn food into energy, and decreased volume of fatty acids, which are essential for energy storage and metabolic processes. These findings also support the involvement of the tryptophan-kynurenine metabolic pathway in CTD-PAH, which has been connected to inflammation and immune responses.
HIF-1α and succinate: Another discovery involved the hypoxia-inducible factor 1α (HIF-1α), a protein that plays a crucial role in cellular response to low oxygen levels (hypoxia). Succinate, a molecule used in energy production, can stabilize the HIF-1α protein, which can lead to increased inflammation when there is low oxygen. From this, the researchers made two main findings:
Seeing these chemical reactions in a patient can mean that their lungs are under high pressure during exercise.
Succinate-induced inflammation may significantly influence how the pulmonary arteries (vessels carrying blood from the heart to the lungs) adjust to changes in blood flow and pressure during exercise.
Why does it matter?
Overall, the researchers found that pulmonary vascular metabolism contributes to CTD-PAH in a dynamic, stress-dependent manner. Even when patients have normal blood pressure at rest, it can increase abnormally with exercise, which is an indicator of this disease. This suggests that future studies need to incorporate exercise in order to successfully examine how the body adjusts energy-producing pathways and oxygen use. The researchers also stress that treatments and therapies aimed at targeting metabolic pathways in PAH should consider these diverse exercise-related changes—and physicians may want to include this transpulmonary plasma sampling method to see what impact the treatment has when the patient is physically active.