Unraveling the Gendered Threads of Brain Aging: Insights from Angiogenesis Markers
By Shreyas Srinivasan
The human brain consists of a highly intricate system of blood vessels known as the cerebral vasculature. It plays a critical role in the functionality of the brain such as providing blood, draining excess interstitial fluid (ISF) and cerebrospinal fluid (CSF). Additionally, this system brings essential nutrients that aids in maintaining the brain’s complex function. Most importantly, these vessels are tasked with bringing oxygen and other nutrients and metabolites from the heart which fuels the nerves, consequently helping send messages from the brain to other parts of the body along with other downstream effects.
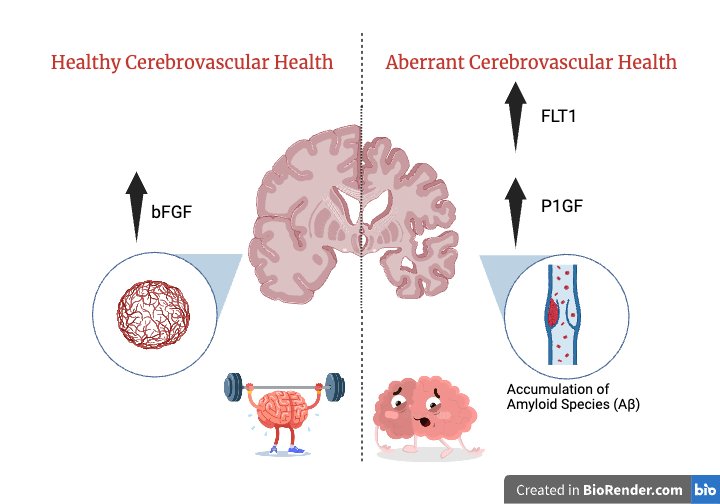
During development, a fascinating process called angiogenesis takes place which is the formation of blood vessels stemming from pre-existing vessels in the brain. One of the many interesting characteristics of angiogenesis is the ability to form new cerebral vessels as needed to sustain physiological functions despite chronic injuries or after acute traumatic events such as strokes. However, this otherwise normal role, can turn abnormal or aberrant leading to cognitive impairment or aging. A new pre-print from Dr. Fanny Elahi’s laboratory at Icahn School of Medicine at Mount Sinai researches the differences between males and females in regards to markers associated with angiogenesis and associations with cognitive aging trajectories.
The researchers in this study predominantly use plasma techniques to measure proteins involved in angiogenesis such as Placental Growth Factor (PlGF), fms related receptor tyrosine kinase 1(FLT1), and Basic Fibroblast Growth Factor (bFGF). With these biomarkers, they find that women show increased vascular risk and men show increased cerebrovascular disease burden. Additionally, they discovered that bFGF decreases with age and that higher levels predict better cognition. This biomarker is particularly helpful in promoting angiogenesis. Hence, as we get older, the levels of these biomarkers decrease, potentially also impairing angiogenesis. Neuroimaging is another technique employed in this study to research the association between brain size (cortical matter) and angiogenesis. Researchers found that aberrant angiogenesis was positively correlated with the burden of white matter hyperintensity which are essentially lesions in the white matter of the brain. These areas could reflect damage in the brain. In regards to gender differences, older men displayed higher levels of AA and lower levels of white matter hyperintensity. Simultaneously, younger men displayed higher levels of AA and higher levels of white matter hyperintensity.
This article brings to light many interesting findings, one of which is that higher levels of specific markers (P1GF and FLT1) indicate pathological conditions in brain aging. Focusing on the gender differences, young women that exhibited higher levels of AA and FLT1 showed higher executive functioning. Another marker that showed remarkable results was Basic Fibroblast Growth Factor (bFGF). This marker has the exceptional quality of promoting neuronal durability in regards to traumatic injuries such as neuroinflammation. So what can we do with this information? One implication this research has is the innovation of diagnostic tools that can measure and monitor markers in the body. Though having an increased level of a certain biomarker doesn’t necessarily constitute increased brain aging, it could provide more insight into how the biomarker affects the brain and its functions. This study also provides potential drug targets which could counteract aberrant angiogenesis. Lastly, similar to using different panels to diagnose cancer, a potential biomarker panel can be created to detect abnormal angiogenesis helping doctors monitor brain health and aging. For example, a biomarker which indicates potential cognitive problems might inspire patients to start exercising, which we know can help keep the brain healthy with aging. Dr. Elahi states, “We are thrilled that this work will enable the development of future sex or gender dependent precision diagnostics and therapeutics in brain aging.”
References:
Agarwal, N., & Carare, R. O. (2021). Cerebral Vessels: An Overview of Anatomy, Physiology, and Role in the Drainage of Fluids and Solutes. Frontiers in neurology, 11, 611485. https://doi.org/10.3389/fneur.2020.611485
Torres-Espin, A., Rabadaugh, H., Fitzsimons, S., Harvey, D., Chou, A., Lindberg, C., Casaletto, K. B., Goldberger, L., Staffaroni, A. M., Maillard, P., Miller, B. L., DeCarli, C., Hinman, J. D., Ferguson, A. R., Kramer, J. H., & Elahi, F. M. (2023). Sexually dimorphic differences in angiogenesis markers predict brain aging trajectories. bioRxiv : the preprint server for biology, 2023.07.16.549192. https://doi.org/10.1101/2023.07.16.549192