Cracking the Code: How Glial Signaling Shakes the Barrier
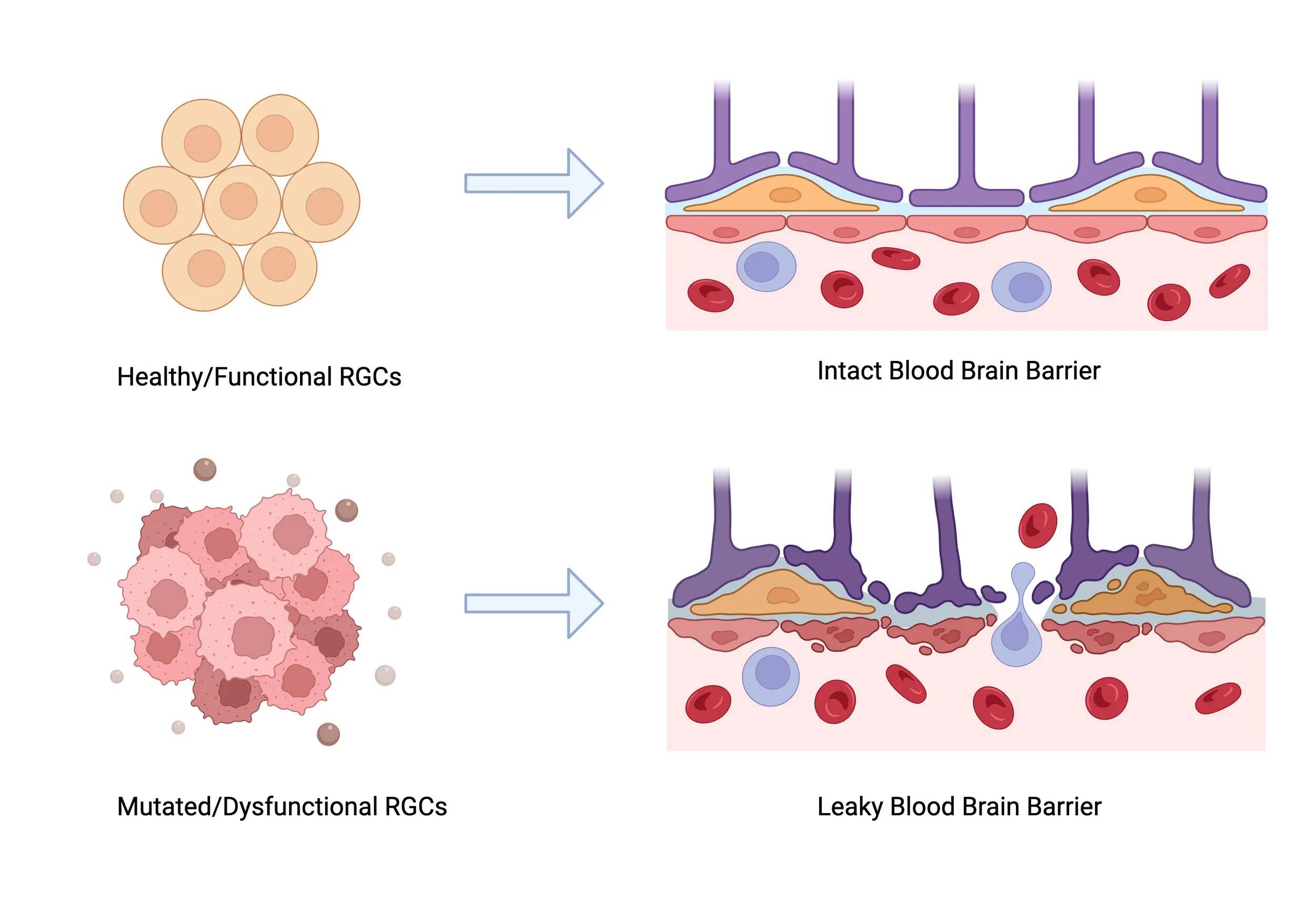
Here in this schema, we see that Radial Glial Cells that are healthy in functioning, help construct a very strong Blood Brain Barrier. However, in cases where Radial Glial Cells are mutated and dysfunctional, a leaky Blood Brain Barrier is caused, resulting in cerebral small vessel diseases such as CADASIL.
By Shreyas Srinivasan
The brain is a complex organ that controls vital characteristics such as cognition, memory, and decision-making. These abilities enable humans to adapt to their constantly changing environments. In order for the brain to perform in a balanced fashion, it must maintain a steady internal environment, known as homeostasis. Hence, the brain attains homeostasis through mechanisms such as: regulating cerebral blood flow, glucose metabolism, a balanced secretion of hormones and neurotransmitters, and preserving proper immunity against viruses and bacteria through the maintenance of the Blood Brain Barrier. Specifically the integrity of the Blood Brain Barrier (BBB) is supported by the Neurovascular Unit (NVU). This unit consists of important cells such as glial cells which are vital in preserving the structure of neurons within the central nervous system. Additionally, this unit helps control the amount of blood flow that enters the brain. However, when this system breaks down or starts to “malfunction,” serious neurological disorders can occur. One such condition is Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL), which is a rare genetic disease that causes the buildup of protein in the blood vessels located in the brain. As a result of the buildup, not enough blood enters the brain due to the blockage, eventually leading to other issues such as strokes and even death. CADASIL can be used as a test case to understand how the pieces of the NVU maintain brain function and prevent disease.
This blog will summarize the article “Endolysosomal dysfunction in radial glia progenitor cells leads to defective cerebral angiogenesis and compromised blood-brain barrier integrity.” While the title is heavy with jargon, the study explores how fundamental cellular functions contribute to larger developmental processes in the brain. Two of the main processes they investigate are angiogenesis (the development of new blood vessels) and the formation of the blood-brain barrier (BBB), a protective barrier that controls what enters the brain from the bloodstream. To explore these processes, the researchers focus on endolysosomes, which are organelles that are important in cellular degradation and maintaining functional and stable proteins in the brain - key to understanding many cellular processes in brain development. The authors study these mechanisms in zebrafish which offer advantages such as rapid development, making them perfect for observing changes in the brain over time.
This study primarily focuses on a gene called Scarb2A. Scarb2A is essential in maintaining proper endolysosomal acidification, which in turn ensures the correct cleavage (division of cells) and activation of the Notch3 protein. In zebrafish without Scarb2A, the acidification of the endosomes are impaired, consequently affecting the splitting of the Notch3 gene. This experiment also reveals that over acidification leads to uncontrolled neurogenesis (growth of neurons) and reduced glial differentiation (stem cells develop into glial cells). Furthermore, this experiment demonstrates dysfunctional vascular endothelial growth factor (VEGF) and dysfunctional Wnt Signaling Pathways, causing: hemorrhages (bleeding), leaky BBB, and several other vascular defects. As a result, Scarb2A mutated zebrafish exhibit excessive differentiation, therefore affecting the activation of VEGF and Wnt signaling pathways. This dysfunction ultimately causes abnormal CNS cerebrovascular development such as a compromised Blood Brain Barrier. To prove this point, experiments show that when a healthy form of Scarb2A is given to zebrafish, normal cerebrovascular development is seen.
Using advanced genetic techniques such as gene manipulation, researchers show that when Notch 3 activity is restored inside a cell, oligodendrocyte precursor cells (OPC) and astrocyte populations are regained; furthermore emphasizing the important role Notch3 plays in the maturation of the cerebrovascular system in the brain. Additionally, in this paper, mutated Scarb2A zebrafish exhibit abnormal branching of the central arteries, alongside a compromised blood brain barrier, and reduced expression (downregulation) of the Glut1 gene (helps with cells receiving glucose, which is the main source of energy).
In conclusion, this article highlights the vital function Scarb2A plays in maintaining endolysosomes function within radial glial cells (RGCs) and the downstream effects it has on Notch3 signaling, cellular differentiation, and overall cerebrovascular development within the central nervous system. Researchers investigated the roles of Scarb2a and Notch3 in cerebrovascular development using zebrafish models. Scarb2a, a protein found in the membrane, is crucial for maintaining the proper functioning of endolysosomes. The study found that mutations in Scarb2a disrupted endolysosomal acidification, impairing the cleavage signaling of Notch3 receptors. This impairment led to a decreased production of the Notch3 intracellular domain (N3ICD), a key component in the Notch signaling pathway, that regulates cell fate in radial glial cells (RGCs).
As a result, there is an imbalance that induces excessive neurogenesis and aberrant glial differentiation. This imbalance negatively affected the secretion of vascular endothelial growth factor (VEGF) and Wnt signaling pathways, both vital for proper vascular development and blood-brain barrier (BBB) integrity. Consequently, the zebrafish models exhibited defective cerebral angiogenesis, micro-hemorrhages, leaky BBB, and compromised myelination (formation of myelin sheath in neurons that allows for quicker neuronal firing).
This paper furthermore highlights the vascular mechanisms in which neuropathologies occur such as Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and how dysfunctional Notch3 can lead to such severe neurological disorders. Understanding these complex mechanisms and the relationship between Scarb2A/Notch3 and cerebrovascular defects, could promote further research into the development of interventions that could potentially reduce the effects of a mutated Scarb2A gene.
The results derived in this study are crucial for understanding diseases such as CADASIL, as they show that Notch3 dysfunction in non-vascular cells like RGCs can contribute to the vascular abnormalities. This expands the current perspective that primarily focuses on vascular cells, and highlights that the cause of CADASIL can also occur due to defects within the other cells within the vascular environment. Further, this study also emphasizes that restoring Notch3 expression and function in RGCs can mitigate the defects caused by CADASIL, bringing to light a potential intervention to this disease.
With such advanced research studies, it is of utmost importance than ever to emphasize how these results translate to real-world impact. Research like this doesn't solely advance our understanding of molecular mechanisms of such diseases, rather it establishes the foundation for helping people live longer and healthier lives.
A link to the full publication can be found here: https://www.nature.com/articles/s41467-024-52365-8