Tracing the Impact of Maternal Hyperglycemia on Fetal Development
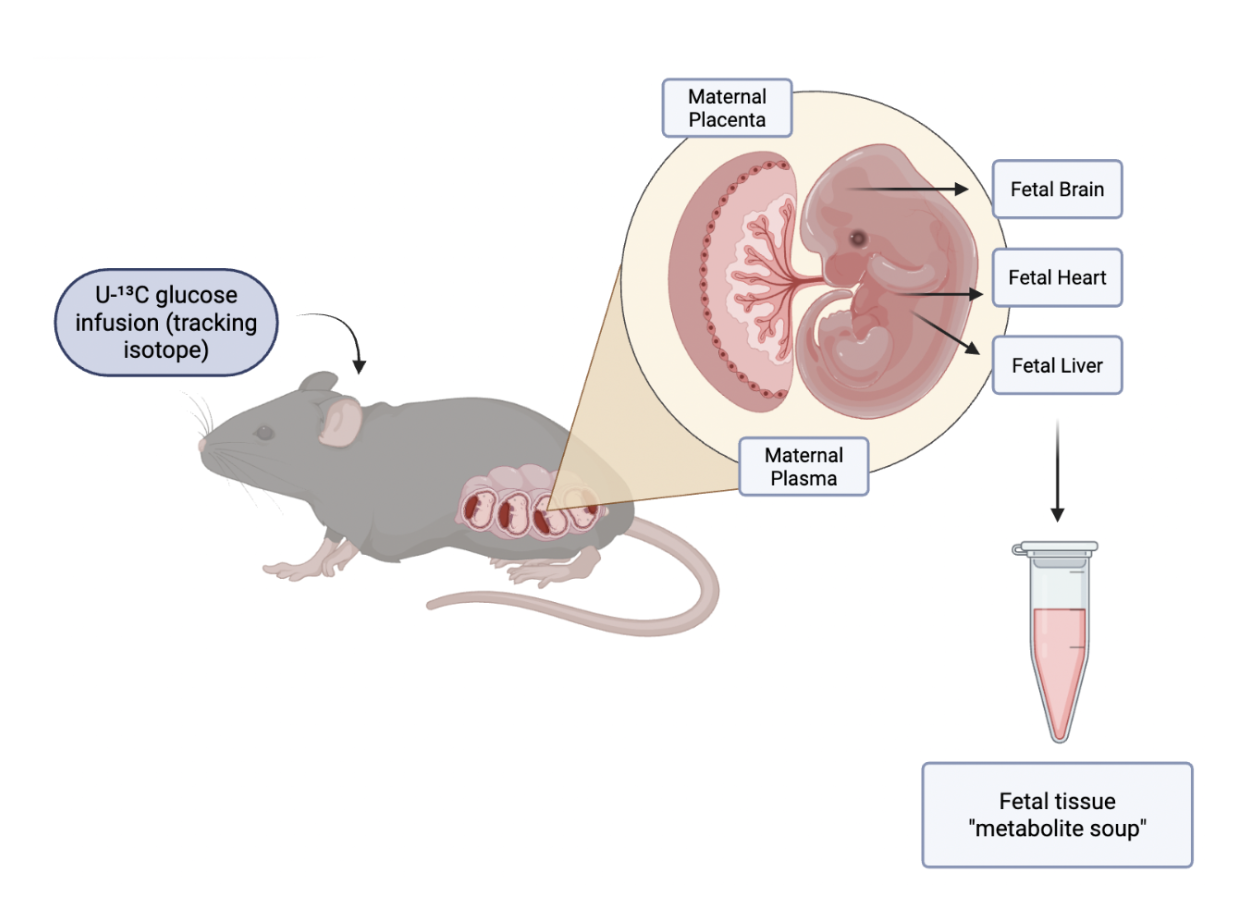
Researchers used Akita mice, a Type 1 diabetes model, to study how high blood sugar in pregnancy affects fetal development. After fasting, pregnant mice received U-¹³C glucose so the team could trace how glucose was metabolized in both mother and fetus.
Written by Yash Sehgal, Schematic by Lexi Bean
In Atlas of fetal metabolism during mid-to-late gestation and diabetic pregnancy, researchers from the University of California, Berkeley set out to answer a key question: how does high blood sugar during pregnancy affect the developing fetus? Although the link between maternal nutrition and fetal health is long-established, the impact of abnormal maternal metabolism on the development of the fetus—particularly during mid-to-late pregnancy—is not well understood. Researchers aimed to fill this gap by using a combination of isotope tracing and liquid chromatography-mass spectrometry (LC-MS) to map how glucose is metabolized in healthy and diabetic mouse pregnancies.
The team used Akita mice, a genetically altered model for Type 1 diabetes, to track the effects of a hyperglycemic pregnancy on fetal development. The Akita dams were bred with wild-type males, and a normal blood sugar control group was also generated by breeding wild-type dams and males. After fasting overnight, the dams were injected with an infusion of U-¹³C glucose—a tracking isotope in which all six carbon atoms in the glucose molecule are Carbon-13—allowing researchers to trace the metabolism of glucose in the maternal and fetal tissue.
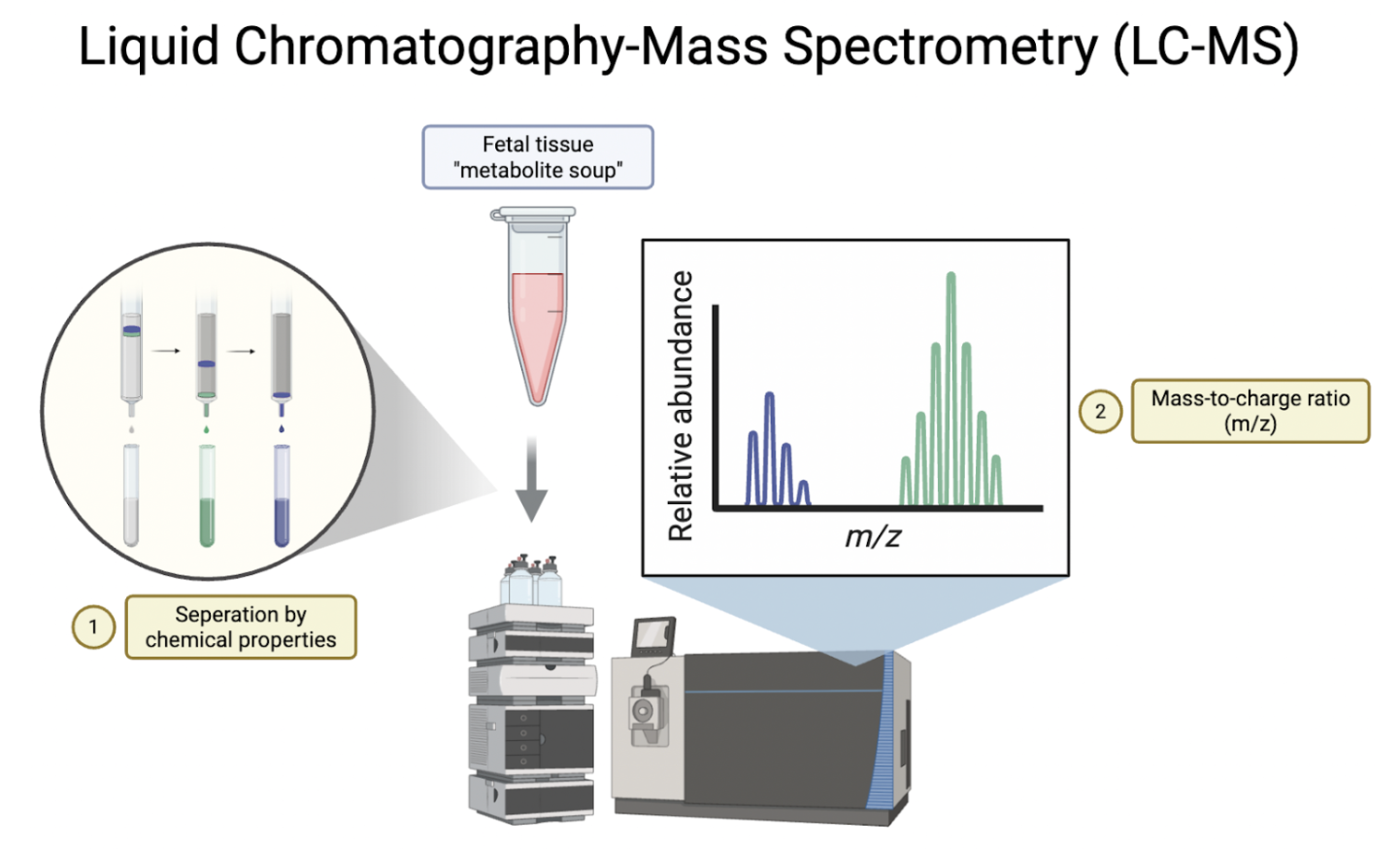
Thirty minutes after the tracer infusion, researchers performed a c-section surgery on the dams and extracted the fetal heart, liver, and brain, as well as maternal placenta. These tissues were flash-frozen to maintain their integrity and to prevent post-mortem metabolic changes. Next, researchers extracted metabolites (chemicals produced during metabolism) from the fetal and maternal tissue for LC-MS.
LC-MS is a two-step technique. In step one, namely liquid chromatography, the metabolite soup that researchers extracted from the fetal tissue is separated based on its chemical properties. The second step in LC-MS, mass spectrometry, measured the mass-to-charge ratio of each metabolite by ionizing metabolite particles and accelerating them through an electromagnetic field. This step tells researchers three things: the metabolite’s identity, how much of it is present, and whether or not it was tagged by ¹³C.
Researchers analyzed hundreds of metabolites, but four main findings stood out.
Altered levels of glycolytic pathway intermediates, such as glucose-6-phosphate.
Fetal brains and livers were found to have high levels of sorbitol buildup, a sugar alcohol that researchers believe has a role in diabetes.
Altered levels of amino acids and their derivatives, including GABA, glutamate and aspartate, were found in the fetus tissues. This could be a cause of neurodevelopmental abnormalities.
The synthesis of nucleotides, the building blocks of genetic material (DNA and RNA), was slowed down in later gestation in the Akita dams.
This study led to some interesting conclusions. First, it showed that fetal tissues are not protected from high maternal glucose. Even when structural abnormalities were not present, researchers noted widespread metabolic disruption. For example, this study found that sorbitol build up could be the underlying factor that causes complications in babies born to diabetic mothers. Sorbitol accumulation, particularly in the fetal brain and liver, is thought to be a potential pathway for cellular damage. This study also suggests that, due to reduced levels of amino acids such as GABA, glutamate, asparate—which are essential to brain development—maternal hyperglycemia may lead to abnormal fetal brain development and function. Lastly, the slower synthesis of nucleotides (building blocks of DNA and RNA) in fetuses exposed to a hyperglycemic environment, particularly during the late gestation period, could lead to abnormalities in cell division and organ growth.
A link to the full publication can be found here: https://pubmed.ncbi.nlm.nih.gov/38070508/