Investigating In Utero Treatments of Hemoglobinopathies
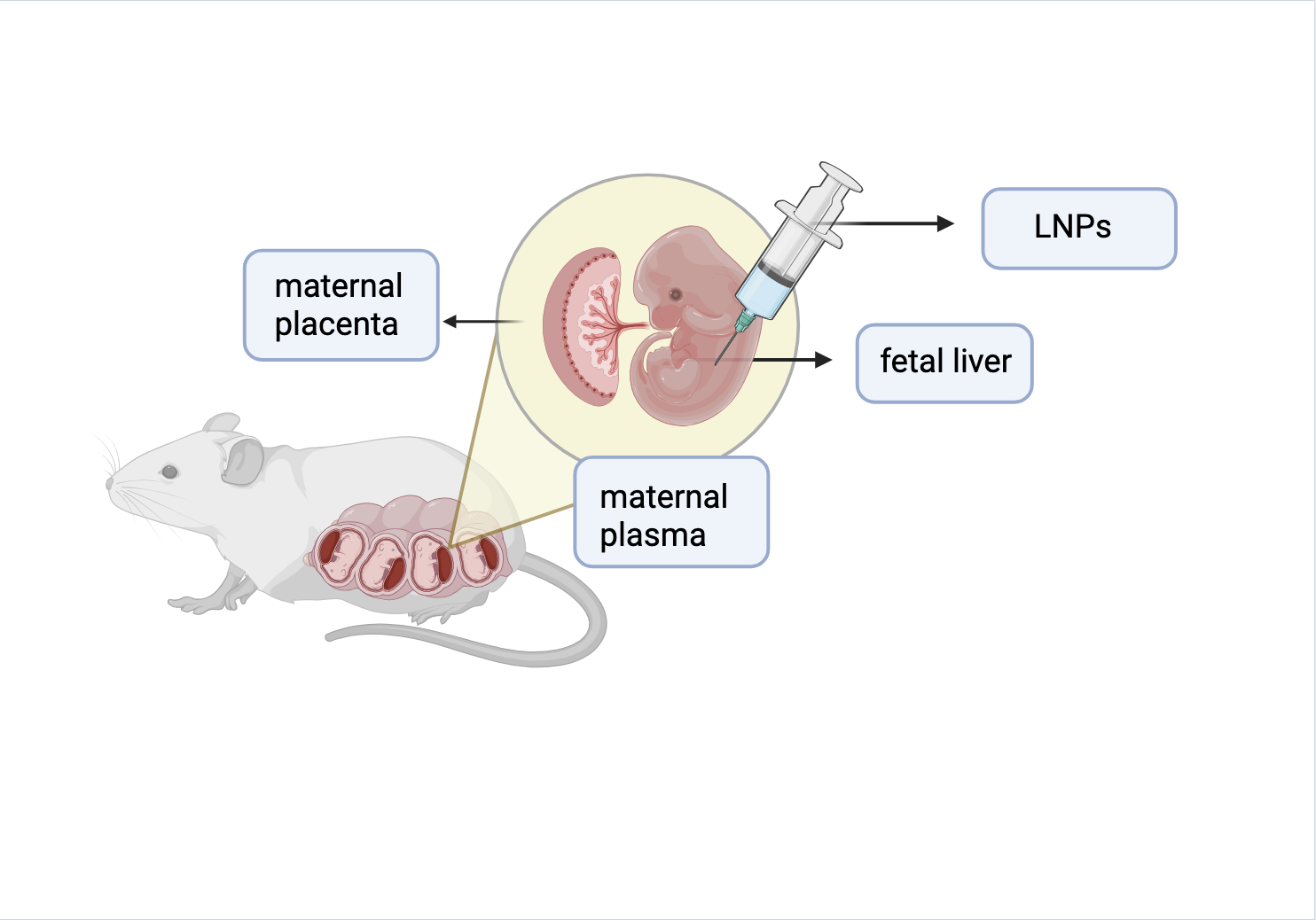
Researchers injected LNPs in fetal mice to target HSCs found in the liver during this developmental period. Their goal was to deliver genome editors and monitor the genetic changes in HSCs.
By Neha Sharma
Hemoglobin serves as the carrier of life, transporting oxygen within our bloodstreams. Without it, mammals would cease to exist. However, this life-sustaining protein can become dysfunctional, causing a variety of disorders classified under the general umbrella of hemoglobinopathies. Hemoglobinopathies are a group of genetic disorders that involve a problem with the production or structure of hemoglobin, most famously including sickle cell disease. Fortunately, there is new emerging research by Dr. Mackenzie and her team that investigates gene editing during fetal and neonatal stages of development that could potentially become treatments for severe, early-onset hemoglobinopathies.
Current treatments of hemoglobinopathies focus on ex vivo (treating tissue outside the body, then transplanting it back) delivery of genome editors, which results in high cost and transplant-related morbidities. Genome editors allow researchers to make precise and deliberate changes to an organism's DNA. Researcher Dr. Mackenzie and her team sought to create an in utero (within uterus) delivery method instead. This method allows for the administration of therapies directly to the fetus, taking away the need for transplants later in a person’s life, thus lowering the risk of later procedures. In two stages, the team identified both an effective delivery tool and the developmental time windows where the treatment works best.
First, they injected fetal mice with lipid nanoparticles (LNPs). The lipids in LNPs are used to encapsulate drugs and deliver them to cells. Using this principle, the researchers at Dr. Mackenzie’s lab realized they could target HSCs in the same way. Hematopoietic stem cells, or HSCs, are stem cells located in the bone marrow. They are responsible for producing red blood cells, which all contain hemoglobin. LNPs encapsulate messenger RNA (mRNA) that contains instructions for editing the DNA in the HSCs. This includes Cre recombinase, which modifies specific DNA. Taking advantage of the fact that HSCs are located in the liver during fetal development, LNPs serve as a good delivery option due to their tendency to accumulate in the liver. This is opposed to accessing HSCs in bone marrow, where they reside for the remainder of life after fetal development. Accessing them requires invasive surgical procedures, which makes injecting fetal mice an easier way to reach and edit HSCs. As a result, LNPs were effective in not only reaching the HSCs but also successfully introducing edits to the DNA, in which the stem cells were still able to differentiate normally afterwards.
In the second stage of their study, they sought to compare the efficiency of gene editing in fetal versus neonatal rodents. This comparison helps determine what the most optimal window for gene editing is and whether it is possible to edit HSC genes after birth as well. They used LNPs to deliver Cas9 (modifies DNA) mRNA and gRNA (guide RNA) to HSCs in fetal and newborn mice. When the fetal mice became adult mice, their HSCs migrated from the liver to their bone marrow. From there, 15-30% of their HSCs still retained the edits made in utero, confirming the persistence of gene edits until adulthood. Similarly, newborn mice that had in vivo (within organism) delivery still exhibited editing changes, but not to the same degree as the in utero delivery. These results indicate that in vivo edits are possible but not as optimal for gene editing compared to in utero.
As the interest in diagnosing and treating prenatal diseases grows, this paper is a great first step in exploring one-time treatments for early-onset hemoglobinopathies. The results from this paper provide evidence for not only successful gene editing in HSCs of a mouse’s fetus but also the potential to edit HSCs early in neonatal life after they are born. Dr. Mackenzie and her team’s findings are a significant step forward in hemoglobinopathy therapy research in finding ways to potentially eliminate the need for bone marrow transplants later in life.
A link to the full publication can be found here: https://pubmed.ncbi.nlm.nih.gov/40463054/